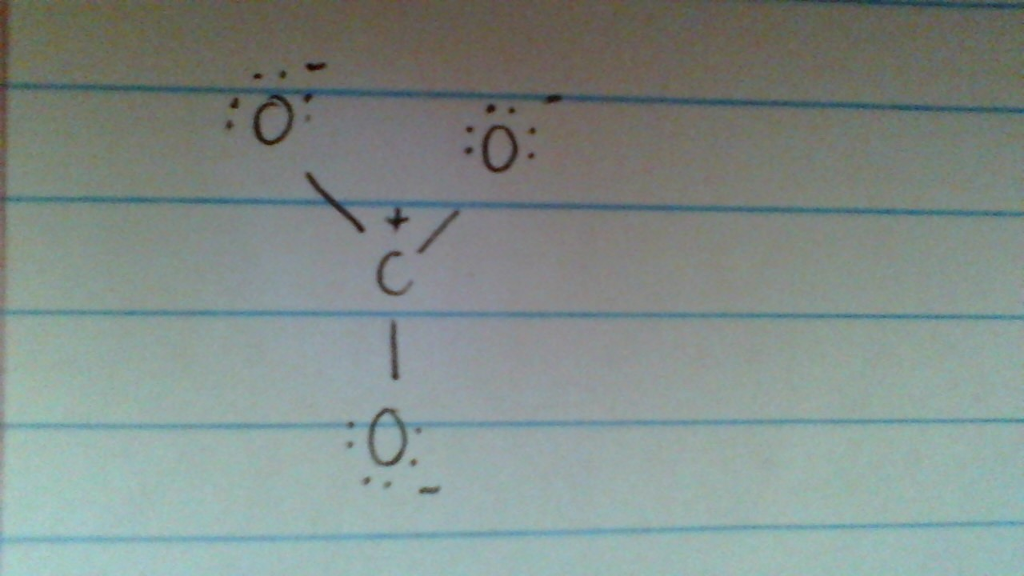
Solved IS THIS A POSSIBLE RESONANCE STRUCTURE FOR CO32??
1. The central atom, sulfur, contributes six valence electrons, and each fluorine atom has seven valence electrons, so the Lewis electron structure is. With an expanded valence, that this species is an exception to the octet rule. 2. There are six electron groups around the central atom, each a bonding pair.
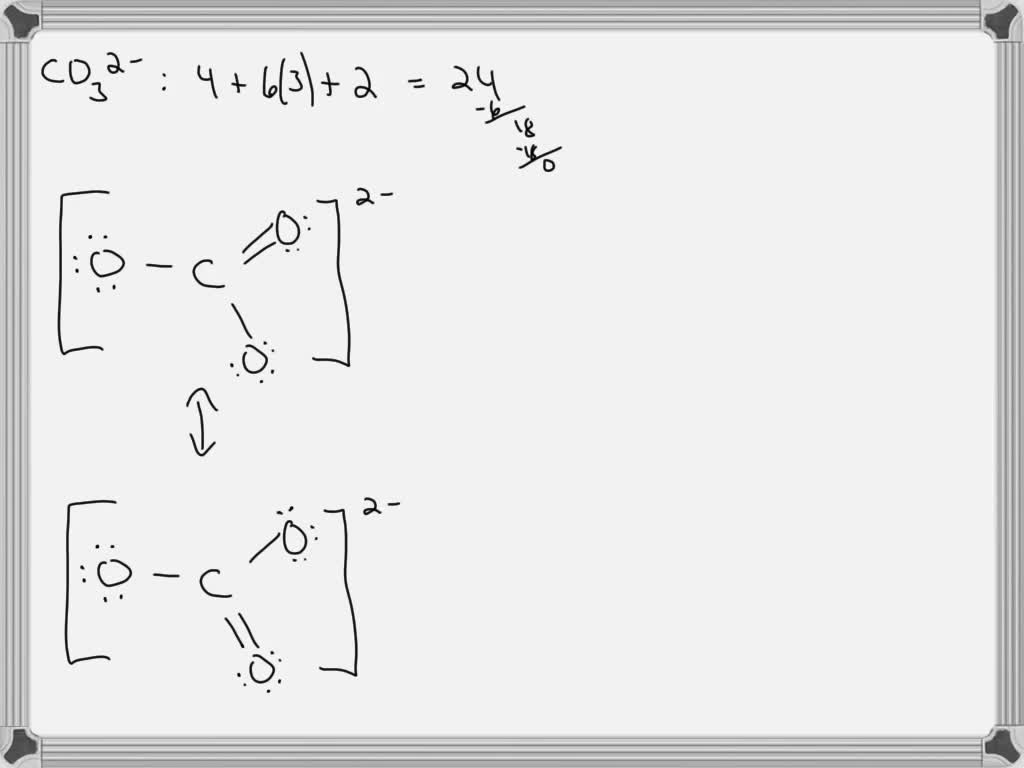
SOLVED For CO32 , carbonate ion, draw the Lewis structure (by
Lewis Structure for CO 3 2-| Carbonate ion. Lewis structure of carbonate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of CO 3 2-.After finishing the lewis structure of CO 3 2-, there should be a -2 charge and it should be stabile structure.
SOLVED Lab 9 Bonding and Molecular Shapes Compound Lewis StructureN
This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. This video discusses the resonance structu.
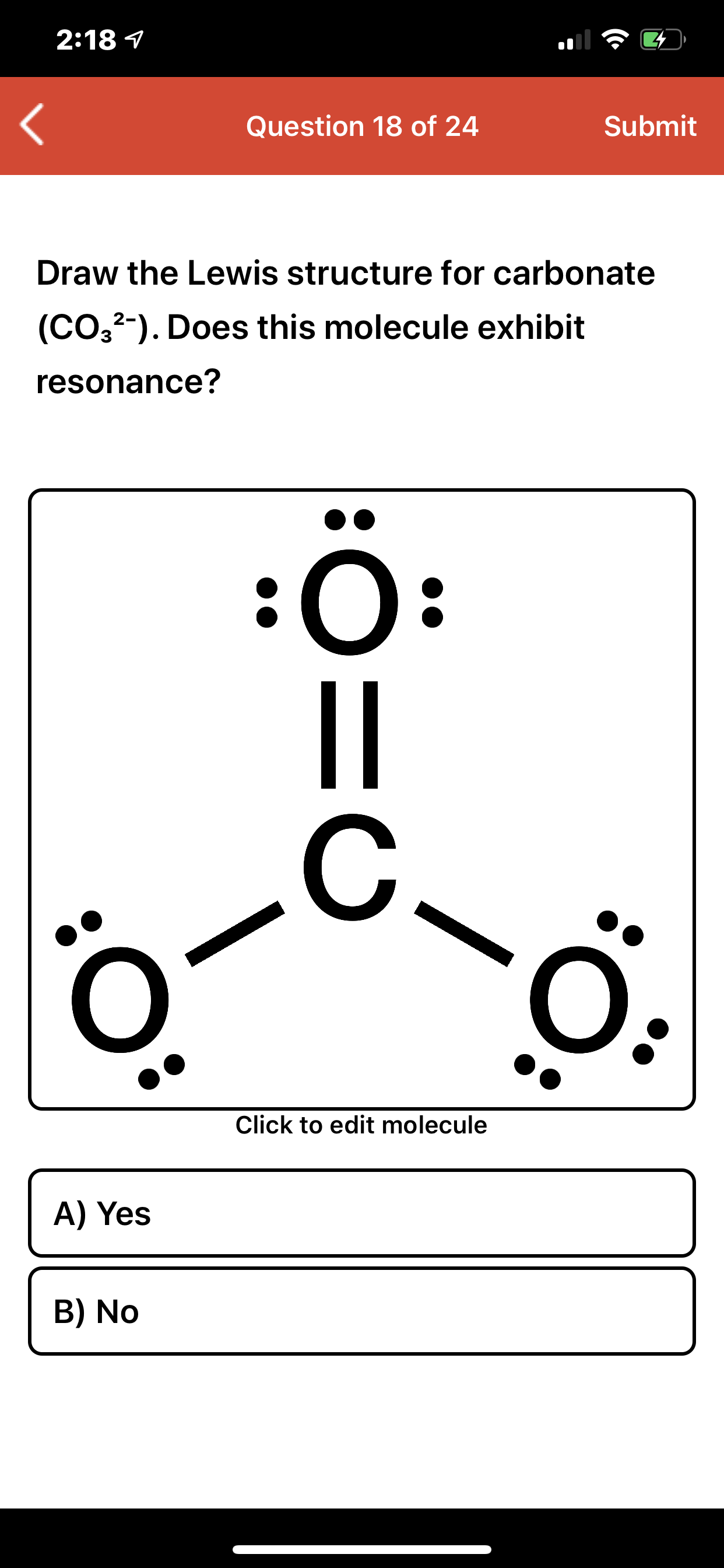
Answered 218 1 Question 18 of 24 Submit Draw… bartleby
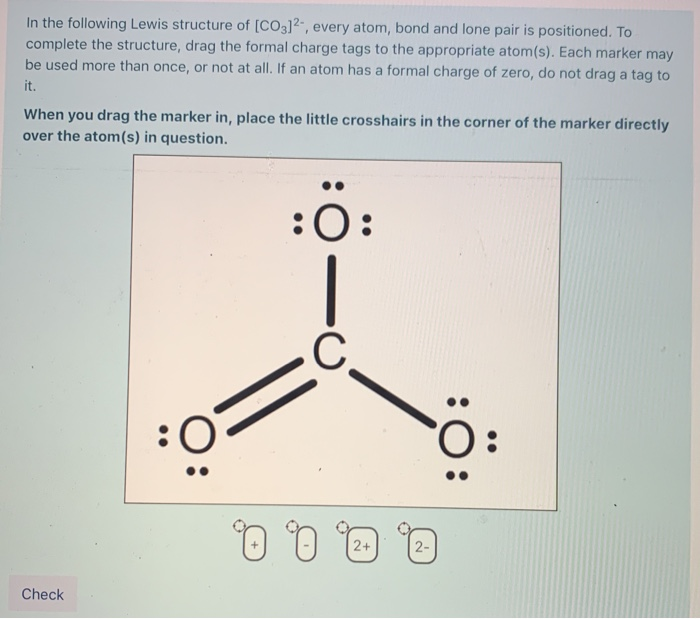
Answer link. The answer is 3 May i recommend a video () Let's consider the Lewis structure of the carbonate ion, CO32‐ . The correct Lewis structure for this ion has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are neutral.
Lewis Structures
The CO32- Lewis structure represents a carbonate ion consisting of one carbon atom and three oxygen atoms. The carbon atom forms one double bond and two single bonds with the three oxygen atoms. Among these, the oxygen atom that shares a double bond has two lone pairs, while the two oxygen atoms that share single bonds have three lone pairs each.

Number of Lone Pairs and Bonding Pairs for CO3 2 (Carbonate ion) YouTube
Since carbon is located in period 2 it does not have access to the d sublevel and must adhere to the octet rule. There are three different possible resonance structures from carbonate. Each carbon oxygen bond can be thought of as 1.333 bonds. the average of a double bond and 2 single bonds. 4 bonds/3 structures. Lewis Dot Structure of CO3 2.
No,No2,Co32 ,lewis dot structure
CO32− . Example 6; Summary; Key Takeaway. With two S=O double bonds, only two oxygens have a formal charge of -1, and sulfur has a formal charge of zero. Lewis structures that minimize formal charges tend to be lowest in energy, making the Lewis structure with two S=O double bonds the most probable. Yes. This is a reasonable Lewis.

What is the Lewis structure of \ce{CO3^{2}} ion? Quizlet
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

CO3 2 Lewis Structure (in 6 Steps With Diagrams) Study Striver
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Lewis structure of CO3 2 ion YouTube
A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion).For the CO3 2- structure use the periodic table to find the total nu.

How To Draw The Lewis Structure of CO3 2 (Carbonate Ion) Chemistry
total valence electron number in CO32- is. = 4 + 6*3 + 2. = 24. Step 2: Determine the Central Atom of the Molecule. Now, in order to draw the Lewis Structure, we have to determine which one is the central atom in a multiatomic heterogeneous molecule, here an ion. In carbonate ion, among the two elements, Carbon has an electronegativity value of.
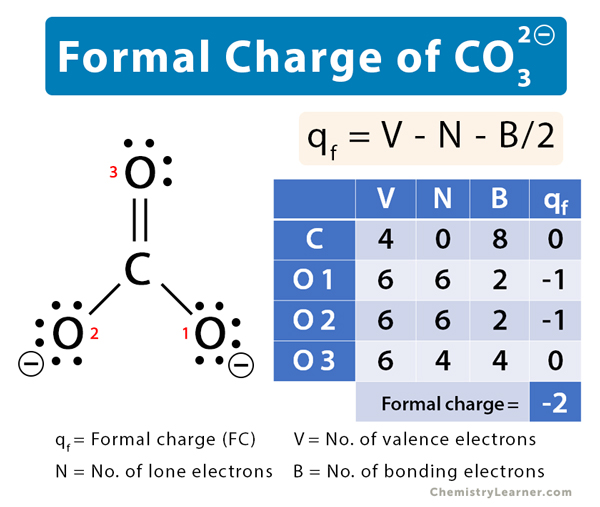
碳酸盐(CO32)形式电荷 188金宝慱手机app
The Lewis structure of carbonate [CO3]2- ion is made up of a carbon (C) atom and three oxygen (O) atoms. The carbon (C) is present at the center of the molecular ion while oxygen (O) occupies the terminals, one on each side. There are a total of 3 electron density regions around the central C atom in the Lewis structure of [CO3]2-.

CO32 Lewis Structure, Characteristics 13 Facts You Should Know
The CO32- lewis structure, it is a diatomic anion, in which only two element are present that is carbon and oxygen atoms. Carbon atom do lies in 14th periodic table group and oxygen atom lies in 16th periodic table group. Thus they both contain 4 and 6 valence electrons respectively. Let calculate the total valence electrons present on CO32.
Identify the correct Lewis structure for CO32. O O 2 O O O O
Draw the Lewis structure of the carbonate ion, CO 32−. (Assign lone pairs, radical electrons, and atomic charges where appropriate.) Calculate the electrons required (ER), valence electrons (VE), and shared pairs (SP). Show transcribed image text. Here's the best way to solve it.

[download 35 ] Possible Resonance Structures For Co32 Free Nude Porn
For the CO 32- Lewis structure there are a total of 24 valence electrons available. Transcript: Let's do the CO3 2- Lewis structure: the carbonate ion. Carbon has 4 valence electrons; Oxygen has six, we have 3 Oxygens, and this negative 2 means we have an extra two valence electrons. Add that all up: 4 plus 18 plus 2: 24 valence electrons.
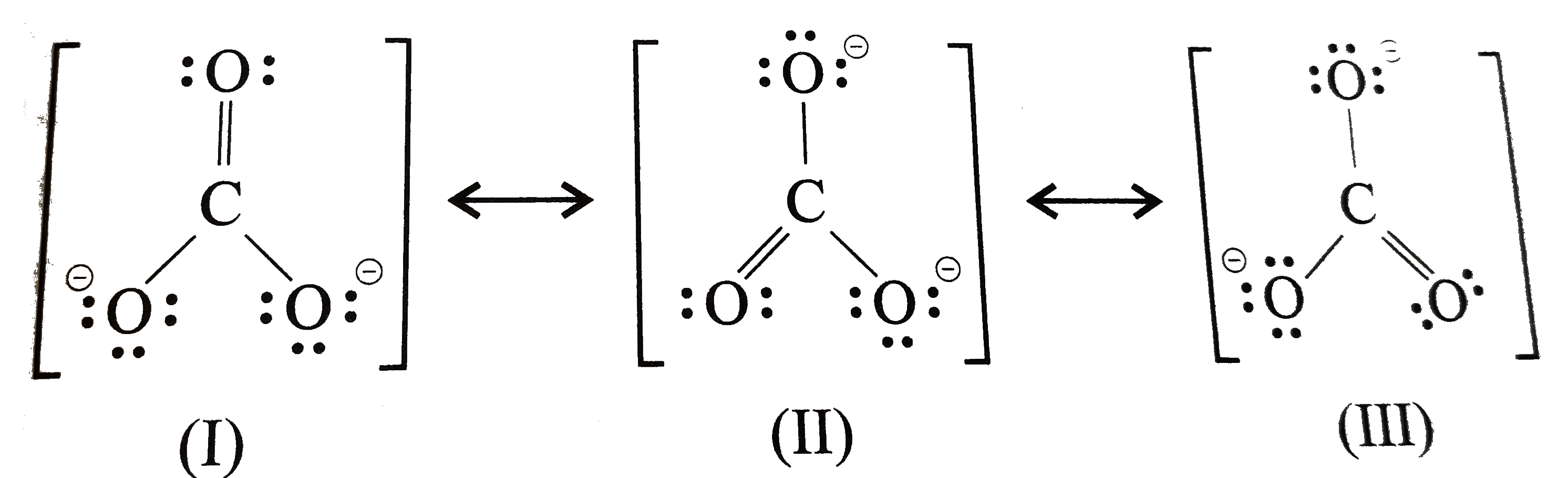
[download 35 ] Possible Resonance Structures For Co32 Free Hot Nude
The CO3 2- Lewis structure is significant as it helps us understand the arrangement of atoms and electrons within the carbonate ion. It provides insights into the bonding patterns, electron distribution, and formal charges, enabling us to predict the reactivity and behavior of CO3 2- in various chemical reactions and applications.